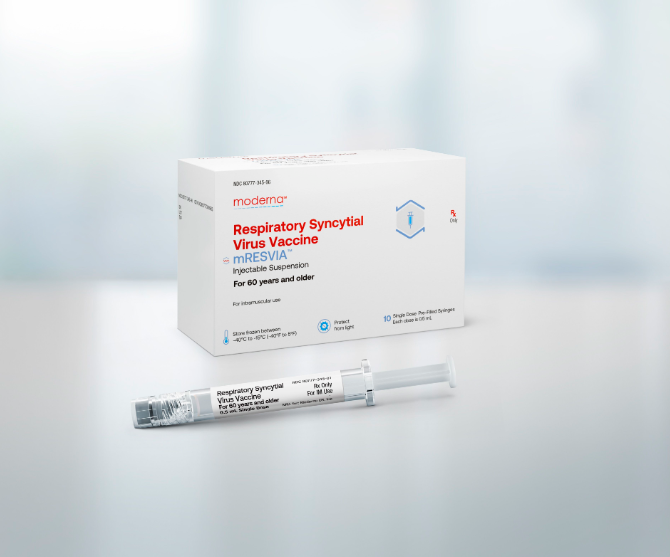
US regulators authorize Moderna’s vaccine against RSV, a respiratory illness primarily affecting elderly and infants
Moderna’s vaccine, mRESVIA, has received approval from the US Food and Drug Administration for use in adults aged 60 and above. This marks a significant step for the company as it diversifies beyond COVID-19 vaccines. Competing with GSK and Pfizer’s RSV shots, Moderna enters a market that generated over $2.4 billion in sales last year. However, its vaccine’s effectiveness showed a slight decline, which could impact its competitive edge against its rivals.
Leave a Reply